



Author: David Weber /
President / Executive Engineering
Date: 8/01/2007
Note:
For out purpose we have over simplified thermal power conversion technology, as
it has nothing
to do with how a Nuclear Accelerated Generator works.
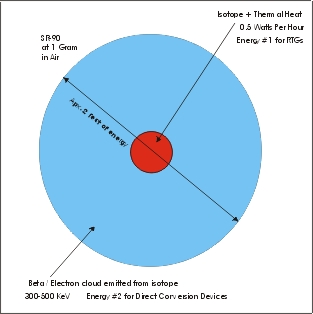
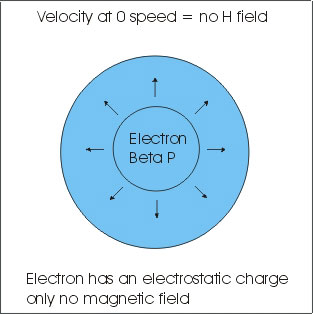
First we need to start with the old and add to the new. The old is the red in the center of the picture above. On a normal hunk of isotope you have heat generated by the movement of the atoms. In this case of SR-90 high energy atoms and lots of them. So 1 gram of SR-90 produces about 0.54 watts of heat all the time. The old and out dated nuclear people use this just like an atomic power plant. They hook up a bunch of thermal couples and you have some electric current. "SO WHAT!" that's the big deal ? Now that red area turns out to be about a 3-10% efficiency conversion using thermocouples, not such a good efficiency.
The thermal nuclear guys only used a small fraction of the energy the isotope is emitting! This is where the big deal is!
So where is the rest of the energy? In the BLUE AREA the thermal nuclear guys see this as wasted energy, when in fact this is where most of the energy is.
So why don't they use this energy? Well until now no one has been able to figure out a way of capturing that energy and use it in a meaningful way.
How we calculate the energy! So you start with some knows. You know that the red area is energy you also know the blue area is energy. So if you add the blue and the red together you get the full area of energy.
So how much energy is in that blue area? That's a good question and it takes some time to get the answer.
|
NOTE:
For you Nuclear Physics Engineers that have not had electronics
BETA PARTICLES
are Electrons.
For you Electrical Engineers that have not had nuclear physics
ELECTRONS are
BETA PARTICLES
!
|
Here are some of the know information you need.
1. SR-90 emits 300 - 540 KevDC beta / electrons.
2. 1 gram
of SR-90 is how many curies? 138 curies per gram of SR90 and
we are going to assume this is a fully emitting gram.
Starting Point of Isotope 138 curies,
1st Half-Life of isotope is ~28 years (this is the isotopes half power point)
this is the first ending point ~28 years later = 69 curies.
All Isotopes have at least 10 half lives so the
69 curies then becomes 34.5 for another 28 years and so on, before they
are considered it a stable material.
3. 1 gram of pure SR-90 = 0.54 watts per hour of thermal heat.
4. How many emission of electrons are there that is measured in a curie? 1 curie = 3.7 x 10¹º per second of emissions. We now need to change this into an hour. So 138 curies x 3.7 x 10¹º = 5.105 x 10 to the 12 power emissions per second, 60 seconds in a minute 60 minutes in an hour = 3600 seconds in an hour, times the above equation. This is 1.8378 x 10 to the 16 power. That's a big number of emissions per hour.
5. We measure electron power in watt hours.
6. How much power is in a single electron emission, you have to measure that in. Magnetic Field Energy=E²V²/A3 so what does this equation say. In simple terms as the velocity of an atom goes up so does its energy. You take a rock as you carry the rock up some steps so does it collect energy when you drop it, it releases that energy it built up. Standard physics! The equation above is the real heart of the how the power conversion is done. It is also what is termed the EMP pulse from an atomic bomb as the energy is released. The above equation is what generates that big electromagnetic pulse "EMP" from an atomic bomb blast.
7. So where do we go next. Next we need to know just how much energy is in all the electrons. As you see the magnetic field changes with the velocity of the electrons. So we are going to use the average here. That's going to be our 300 KevDC that is 300,000 VOLTS Direct Current for each electron. Since the voltage of the electron is in fact what determines the speed we need to know what that speed is.
8. Electron Velocity
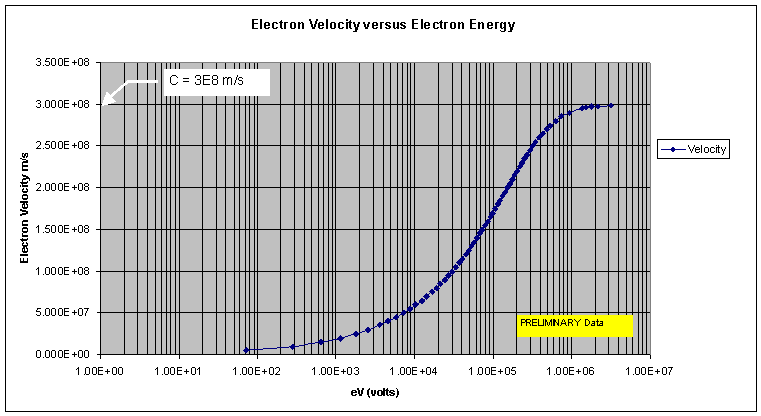
Now that you have the speed of the electron you can now compute the magnetic energy. You will notice that Einstein was correct that as you approach light speed the energy required goes off the chart. This is where all the energy comes from in a BETA isotope. An electron at rest has no energy other than it own electrostatic electrical charge. ZERO speed equals ZERO ENERGY! The trick here is how do you capture this high speed energy. Just so everyone knows the physics guys use meter per second for travel distance. This gives us the "V" so V=1.30E+08 part of the equation. So V² = 1.69 x 10 to the 16 power.
10. So what about that E²? That is the charge of the a particle at rest, where the velocity = 0 of an electrostatic field. So since we are using Beta particle / Electron, that number is 4.80325 x 10 to the -10 power for one single electron measured in electrostatic units "E" at rest, so E² = 2.307121056 x 10 to the -19 power.
11. So what is A3? "A" is the radius of the electron in meters. That number is 2.8179 x 10 to the -15 power. Pretty small electron. So our A3 = 8.4537 x 10 to the -15 power.
12. So now we have all we need for a single electron and how much electromagnetic energy it will produce. So the MFE (H field) = 3.899034585 x 10 to the - 3 power/8.4537 x 10 to the -15 power. MFE (H field)= 4.612222559 x 10 to the 11 power. MFE is the electromagnetic field around a single electron with a 300kev electrical charge. That's a very big number for a single electron.
13. To put this in prospective you need to see some pictures of the MFE or what is referred to the (H field). We also need to know the area this field covers, we are dealing with distances and coupling actions in this case. For those of you that are amateur scientists and you wondered how that magnetic field of a wire is projected so far out from the wire you will find the following information interesting.
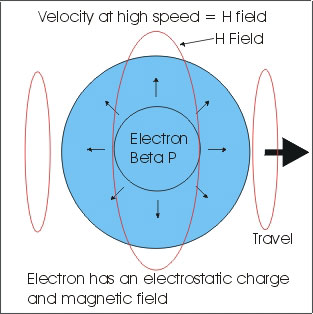
Now I want to point out that the pictures of the H field are somewhat deceiving. The H Field area and distance from the electron is just enormous compared to the size of an electron I mean really really really big! On the order of the electron being earth size the H field would be light years in distance. You need to keep some kind of prospective as to size.
Electron are so small no one knows what shape they are, we think they are round, that in fact many not be the case. In any case the H field covering an area is H/8pi so if we take the number from above. 4.612222559 x 10 to the 11 power / 8pi = 1.835145047 x 10 to the 10 power this is the area magnetic energy (H field). Keep in mind that this is a single electron at 300,000 volts.
14. At this point we can take the magnetic field energy and turn that into power. But before do that we need to look at something else.
15. Where does that 0.54 watts of heat come from in that isotope. Well it comes from atoms smashing in to each other.
16. Their are two forms of energy here, we are dealing with Kinetic Energy, speed of an electron hitting something and Dynamic (H field) Magnetic Energy. We need to look at this in a meaningful way.
17. We need to divide this in two sections Kinetic Energy and Magnetic Energy (H field) so the total energy is this. TOTAL ENERGY= 1/2 M/V²+E²V²/A3
18. Kinetic Energy is done this way. This is where most of the heat of the isotope comes from. Kinetic Energy = 1/2 M/V². Atoms smashing into each other cause most of the heat that is generated in the isotope. So lets look at this Kinetic Energy and find out just how many atoms it takes to do this 0.54 watts of heat energy. Since we have solved for some of this before we have one of the numbers. V² = 1.69 x 10 to the 16 power and the mass of one electron at rest is M=9.10956 x 10 to the -31 power (kilograms). For you purest in physics we know the mass of an electron changes as we approach the speed of light. For what we are doing the electron is almost stopped. So the Kinetic Energy = 2.6951 x 10 to -47 power. We well get this answer at the end of the page.
19. So at this point we are going to switch gears and do some simplification. The fact is that we have emitted particles. We also know they have a know average voltage.
Nuclear Physics Engineers are going to Scream No, but in fact the answer you will see is correct!
The following is taken from physics books.
|
Since a coulomb is approximately equal to 6.24150948×1018
elementary charges,
one ampere is approximately equivalent to 6.24150948×1018
elementary charges, such as electrons,
moving past a boundary in one second. The numbers = 1 Amp of
electrical charge or electrical current (I or AMPS).
|

1 curie = 3.7 x 10¹º per second of emissions. So 138 curies x 3.7 x 10¹º = 5.106 x 10 to the 12 power.
So our isotope has 5.106 x 10 to the 12 power of elementary charges coming from it per second.
So we take 6.24150948×1018 / 5.106 x 10 to the 12 power = 1222387.286
That is 1/1222387.286 of an Amp = 8.180713359 x10 to the -7 power "AMPS" of current in 1 second
To find power we know that the particles are averaged at 300,000 volts
300,000 "volts" x 8.180713359 x10 to the -7 power "amps" = 0.2454214008 Watts Per Second
VOLTS x AMPS = Power in WATTS per seconds
To change this to watt hours you need to X it by 3600, 60 second in a minute and 60 minutes in an hour.
The area in blue of the isotope
produces 0.2454214008 Watts Per
Second, I know you nuclear physics guys are just
cringing at this point yelling how can this be. But, be it must!
Changing
this into a watt hour is 3600
x 0.2454214008 Watts = 883.5170429 Watts Per Hour.
If we use the the 540,000 volts this power
in the isotope increases to
1590.330677 Watts Per hour. Some big
difference!
So our 1 gram of isotope of SR-90 produces between 883.5170429 to 1590.330677 watts of power for our Nuclear Accelerated Generator per hour.
I want to point our that the real world measures power in watt hour or kilowatt hours for the most part!

To explain how an isotope can produce so little heat and yet so much power is easy to explain. But you have to follow the science.
20. So a good point to start is friction, if you slide two solid objects against each other they get hot. Friction between the atoms generates the heat. You have to keep this in mind. Now you electrical engineers will have an easy time understanding this. This Isotope temperature per gram is quite simple, part of the problem is electrical and part of the problem is electro chemical. We have particles of electrons so we have friction in the isotope. If you look at this from an electrical stand point it is similar to ohms law. E = I x R where E is the voltage I is the current and R is the resistance or friction of the isotope. You will also need the to know the power, P = I² x R or P = V x I
This is how the ENTROPY of an isotope thermal system works!
A good analogy to this is wire, all types of wire and electron flow through that wire. Another good analogy to this is semiconductors and in fact most nuclear isotopes that are used for RTGs technology are just that, a semiconductor. Most people will not think of them as a semiconductor but in fact that's what they are. So Why?.
Back to ohm law we go. If you take a foot of copper wire it has resistance. You can use ohms law to calculate that resistance and that power. if we take a 30 volt battery and run a current through a wire that is 10 ohms we get 30 watts if we take that and change that wire so the resistance is 50 ohms we then get 18 watts. If take a wire that is 1000 ohm we now get. 0.9 watts, so why does this look wrong?
Well from that stand point of an isotope it is in fact a type of Constant Current Device. So lets do this with constant current and see what we get. 30 watts for 10 ohms, 50 watts for 50 ohms and 1000 watts for 1000 ohms. So what changed is the voltage in this case.
The driving force of the electrons is voltage, you have to select the right chemicals and or friction of the semiconductor to make the isotope produce its best power for the emissions you have. If you change the conductivity of the isotope in either direction from its optimal heat performance your heat production will go down, if the semiconductor is to high in resistance the heat drops or if the isotopes resistance gets to low the heat drops. You have to get it just right to produce that maximum heat level.
All most no RTGs are built with pure isotope they are for the most part a mixture of chemicals (semiconductor) to generate the most heat.
Conductivity: As to strontium’s electrical and thermal conductivity, the electrical conductivity measured as to electrical resistivity @ 20 ºC is 23 µOcm and its electronegativities (or its ability to draw electrons relative to other elements) is 0.95. The thermal conductivity of strontium is 49 W m-1 K-1. Well it looks like SR-90 is some what conductive.
21. So what is our SR-90 resistance? Back to ohms law, well we have 300,000 volts divided by 5 x10 to the -10 power of AMPS or current = resistance of the isotope is 6.0000 x 10 to the 14 power, by standard electrical or electron terms it has a high resistance per area, so that is where the heat comes from. So why not use a thermal couple to make electric current. Well size and the efficiency of the thermocouple devices. The efficiency of a thermocouple is about 3% to 10% efficient in making electric current, so we have lots of wasted energy. All in all most of us would not pay for the use of such a device because of expense.
So the bottom line answer is this, if we have 0.54 watts (hours) we now need to change this to watt second so 0.54 / 3600 = 1.5 x 10 to the -4 power from our isotope and our electrons are at 300,000 volts, we have a current of 1.4 x 10 to the - 4 power, so 1/1.4 x 10 to the -4 power = 7142.857143 Then one AMP = 6.24150948×1018 / 7142.857143 = 8.7381132272 x 10 to the 14 power. That is the number of electron in the isotopes hot core. This is only the Kinetic Energy (Red Area only) not the emitted energy.
22. Now I want to point out that we now have the Kinetic Energy and Magnetic Energy (H field) energy. The total energy of the isotope is as follows. 0.54 watts = 0.54 watts per hour on the thermal side + (H) field of 1590.330677 watts per hour = 1590.870677 + watts per hour. Their is a big difference between the two types of power.
If the isotope is tweaked for maximum power transfer (semiconductor) then it will transmit more power to a thermal couple. But standing alone as a "Pure Isotope" their is very little power in the way of heat.
23. A few things we need to mention about isotopes, if you were to take an isotope and put a single layer of molecules down on a sheet of foil the ejection voltage of the atoms would all be about 540,000 volts thus changing the power ratio of the isotope in a substantial way. So not only area, but volume effects how the power of the isotope is effected.
24. For those of you that have looked at other atomic battery configurations and seen these huge numbers on power conversion, those number did not come out of thin air in fact their is a process in electronics that is referred to as electromagnetic eddy currents and works on the quantum physics level, these currents in fact may increase the decay acceleration of an isotope increasing it output power but decreasing its half life, no matter what way you look at it, you can not create or destroy energy, only transform it!
On one final note about power conversion: In some cases power can be deceiving if you take 3% thermocouples for our isotopes 0.54 watts that = 0.0162 watts So if you use that number and divide that into our NAG power at 1590 watts at 85% = 1351.5 / 0.0162 = 83425 this number is correct for a power transfer number for what we did, but it is some what deceiving from the point of view of isotope power production as no RTG is build from pure isotope, so those big transfers number are not always correct, you have to compare apples to apples.
25. Since the
Nuclear Accelerated Generator in fact has a 85% conversion efficiency that
1590.330677 watts turns into 1351.781075 watts.
That same power from a thermal device at
10% would be 159.033 watts.
So that 5KW/Kg limit that Nuclear Engineers
thought they had would in fact be 1,590,000 @ 85% = 1,351,500 watts per Kg of
isotope.
Many thanks to those of you that read this far.
Acknowledgments to all that helped:
Dr. Michel Huff (MIT) Mems Organization Nano-Technology
Dr. Mitch Ferren Oak Ridge Nation Laboratories Isotope Preparation
Dr. E LEE Chemical Engineering